( this is sumthing which may b asked in GS Paper III )
What are Lithium Ion Batteries ? What are its Advantages and Disadvantages ?


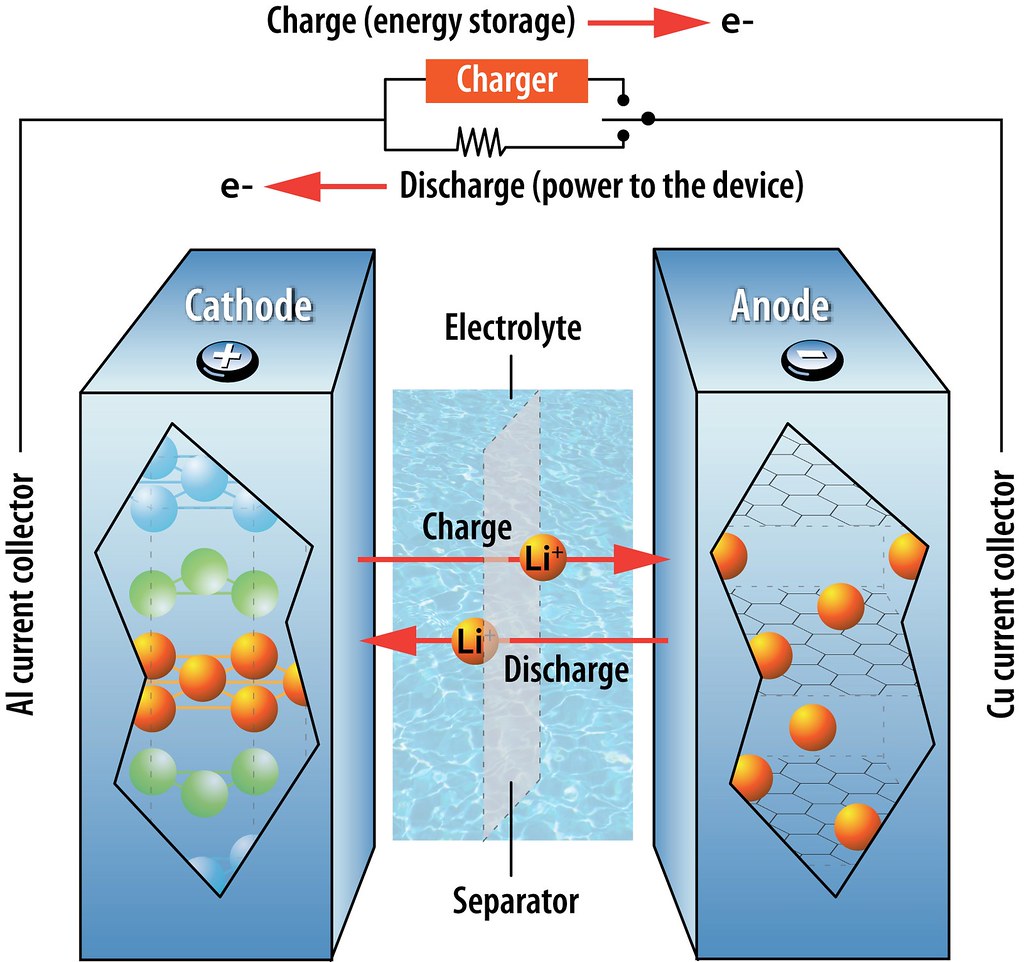

They're generally much lighter than other types of rechargeable batteries of the same size. .
The electrodes of a lithium-ion battery are made of lightweight lithium and carbon.
Lithium is also a highly reactive element, meaning that a lot of energy can be stored in its atomic bonds.
This translates into a very high energy density for lithium-ion batteries.
A typical lithium-ion battery can store 150 watt-hours of electricity in 1 kilogram of battery.
A NiMH (nickel-metal hydride) battery pack can store perhaps 100 watt-hours per kilogram, although 60 to 70 watt-hours might be more typical.
A lead-acid battery can store only 25 watt-hours per kilogram.
Using lead-acid technology, it takes 6 kilograms to store the same amount of energy that a 1 kilogram lithium-ion battery can handle. That's a huge difference
.jpg)
The technology of the lithium battery has been slowly improving to create much more
stable products.
A look at the future in this regard...!
******************************************************
Do u knw

18-year-old Indian-American whizkid Esha Khare who has revolutionized cellphone technology by creating a device that can charge a cellphone super fast in 20 seconds.Called super-capacitor, the device can do up to 10,000 cycles, as against 1,000 that conventional battery chargers do.
What are Lithium Ion Batteries ? What are its Advantages and Disadvantages ?

- Lithium-ion batteries are incredibly popular these days. You can find them in laptops, PDAs, cell phones and iPods. They're so common because, pound for pound, they're some of the most energetic rechargeable batteries available.


- They hold their charge. A lithium-ion battery pack loses only about 5 percent of its charge per month, compared to a 20 percent loss per month for NiMH batteries.
- They have no memory effect, which means that you do not have to completely discharge them before recharging, as with some other battery chemistries.
- Lithium-ion batteries can handle hundreds of charge/discharge cycles.
- They start degrading as soon as they leave the factory. They will only last two or three years from the date of manufacture whether you use them or not.
- They are extremely sensitive to high temperatures. Heat causes lithium-ion battery packs to degrade much faster than they normally would.
- If you completely discharge a lithium-ion battery, it is ruined.
- A lithium-ion battery pack must have an on-board computer to manage the battery. This makes them even more expensive than they already are.
- There is a small chance that, if a lithium-ion battery pack fails, it will burst into flame.
That is not to say that lithium-ion batteries are flawless. They have a few disadvantages as well:
.jpg)
The technology of the lithium battery has been slowly improving to create much more
stable products.
A look at the future in this regard...!
LITHIUM-SULPHUR:
- This rechargeable battery is known for its high energy-storage capacity.
- Scientists say it may succeed the lithium-ion cell because of its efficiency and low cost.
- It is also lighter than lithium-ion. But its success depends on eliminating some of its technical constraints, such as short life span.
- has increased life span
- This electro-chemical battery uses zinc along with oxygen sourced directly from air.
- It has high energy storage capacity and is relatively less expensive.
- It differs in size and ranges from small cells used in hearing aids to larger batteries used in film cameras
BIOCOMPATIBLE ZINC-AIR:
- The technology, commonly found in hearing aids, may soon find application in implantable medical devices.
- Scientists from Institute of Physical Chemistry, Poland, have developed a zinc-air battery with a biological touch.
- They have created an electrode with enzyme bilirubin oxidase wrapped in carbon nanotubes. This specially designed electrode is able to supply 1.6 volts power for one-and-a-half week.
SODIUM-ION:
- It is rechargeable and uses sodium-ions to store energy.
- Though still in its infancy, the battery is set to replace lithium-ion battery soon due to its high efficiency and low cost.
- Salt power in the form of sodium is considered promising.
- Scientists have also turned to sugar to empower their sodium batteries.
- They found that when carbon made from sugar is used in the battery it greatly increases the battery capacity.
SODIUM-AIR:
- It also has high energy storage capacity.
- Sodium is cheaper and more abundant than lithium.
- A study by researchers from Institute of Physical Chemistry in Germany showed that replacing lithium with sodium in metal-air battery improves rechargeability.
- Most lithium-air batteries have limited use because they are single-use and cannot be recharged. This is due to lithium’s instability when combined with air. However, the scientists found that sodium is stable even when combined with air and, hence, can be recharged.
LITHIUM-AIR:
- It has a metal-air battery chemistry which uses oxygen present in the atmosphere for its reactions.
- A lithium-air battery possesses high energy storage capacity because it uses oxygen from the air instead of storing an oxidizer internally.
- Industry experts tout this battery as one of the prominent candidates for future high energy storage devices.
- If commercialised, it may find its primary application in the automotive industry
******************************************************
Do u knw

Khare, who is from Saratoga in Santa Clara in California, has been conferred this year’s Young Scientist Award from the Intel Foundation for her invention. The award carries a cash of $50,000.She said she started working on the energy-storage technology as a solution. Her specialization in nano-chemistry she said , helped her scale down the size of the device.